India has actually prohibited the manufacture and export of 2 extremely addicting opioids– tapentadol and carisoprodol– after a report exposed their function in sustaining a drug crisis in West Africa.
Aveo Pharmaceuticals, based in Mumbai, was discovered to be unlawfully exporting this mix of drugs to nations like Nigeria, Ghana and Ivory Coast.
The BBC covertly taped video footage revealing an Aveo director confessing that their drugs were “extremely damaging” however successful.
The business’s factory was consequently robbed and its stock took.
The Indian health ministry revealed on Sunday it had actually begun the procedure to instantly withdraw export and production licences for the drug mix.
It likewise suspended all operations by Aveo following an audit by the Central Drugs Requirement Control Organisation and the Maharashtra state regulative authority.
” Roughly 1.3 crore tablets and 26 batches of Active Pharmaceutical Components of tapentadol and carisoprodol were apprehended to avoid additional circulation of these possibly unsafe drugs,” the ministry stated.
India’s Food and Drugs Administration pledged more stringent tracking to avoid future prohibited exports and protect the nation’s track record as a significant gamer in the pharmaceutical market.
The Drugs Controller General, Dr Rajeev Singh Raghuvanshi, stated he had actually withdrawn authorization to produce and export the mix due to the “capacity of substance abuse and damaging influence on population”.
The Independent has actually connected to Dr Raghuvanshi for remark.
” Aveo has actually been on our radar for the previous couple of months. We served them observe in October for non-matching of their production and circulation records,” an unnamed authorities from the Maharashtra drug regulator was priced quote as stating by The Times of India paper. “The Central Drugs Requirement Control Company is accountable for checking the exports based on their procedures, and the state does not contribute.”
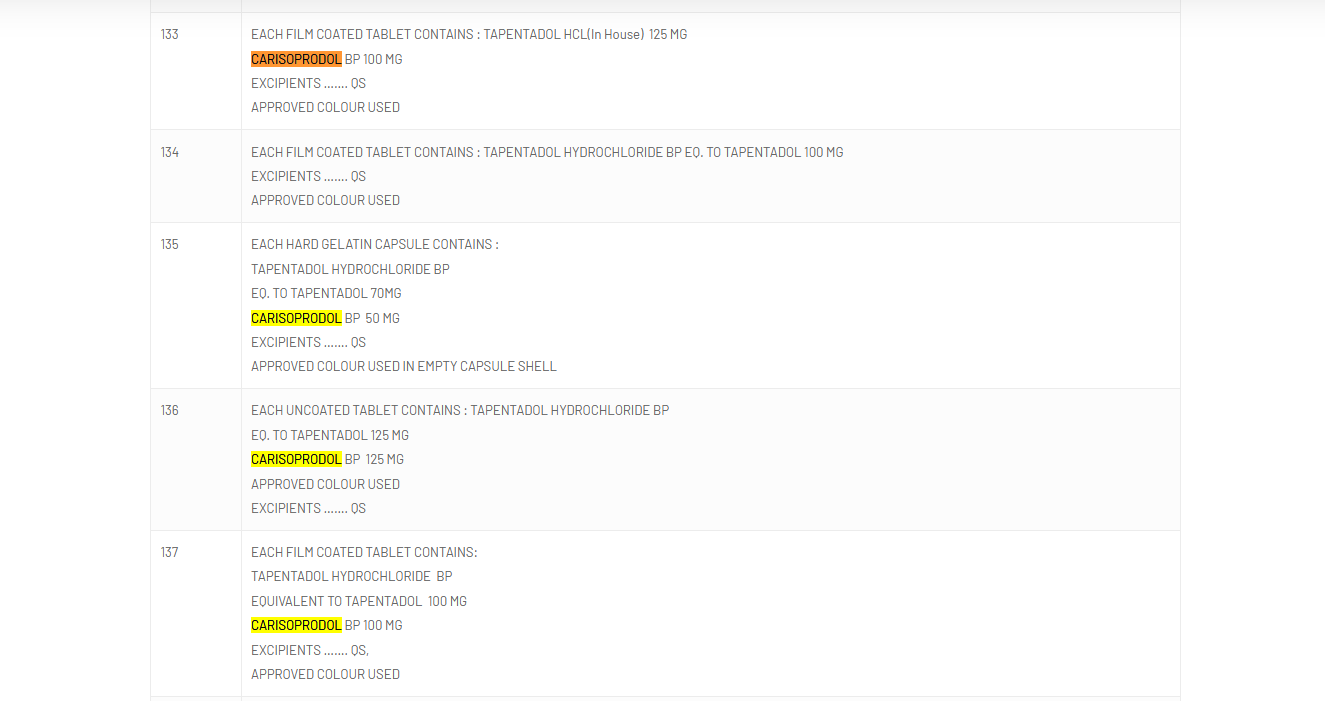
The mix of tapentadol, an effective opioid, and carisoprodol, an addicting muscle relaxant, is not authorized for medical usage due to its serious health threats, consisting of breathing troubles, seizures, and deadly overdoses.
” The medications have their usage independently however their mix is damaging, and it is currently prohibited in India,” the state authorities discussed to the everyday.
However the drug mix has actually flooded West African markets, especially Nigeria, where an approximated 4 million individuals abuse opioids. The World Health Company approximates that around 100,000 individuals in Africa pass away each year due to “falsified or substandard” medication.
The BBC examination discovered that Aveo and its sibling business, Westfin International, had actually delivered countless these tablets to West Africa, where they were honestly offered on the streets.
In the covertly taped exchange, Aveo director Vinod Sharma acknowledged the risks of the tablets. “This is extremely damaging for the health,” he stated. “Nowadays, this is organization”.
When an undercover BBC operative, impersonating an African business person, recommended offering the tablets to Nigerian teens, Mr Sharma responded, “OK.”.
He discussed that taking 2 or 3 tablets simultaneously would enable users to “unwind” and get “high”.
In a declaration on its site, Aveo turned down the accusations levelled versus it as “completely unwarranted and without benefit”. “We have actually constantly stuck to the guidelines and guidelines set by numerous regulative authorities to produce and export our items,” it stated. “Tafrodol is our signed up hallmark, which includes both tapentadol and carisoprodol. This mix is accredited by the appropriate State Fda and is exported under the essential No Objection Certificate from the Assistant Drug Controllers and with an export licence provided by the Central Drugs Requirement Control Company.”.
The nationwide drug regulator, nevertheless, clarified that while tapentadol and carisoprodol were authorized for usage in India separately, their mix was not.
” It is necessary to keep in mind that Aveo Pharmaceuticals isn’t the only business in India producing a comparable mix item. In truth, numerous business are unlawfully utilizing our trademark name and logo design. We have actually currently submitted several legal cases versus such business, and the matter is presently being heard in the High Court,” a representative for Aveo Pharmaceuticals stated. “In addition, the production code of tafrodol blister displayed in the BBC post does not bear our production code of our factory portraying it has actually been produced by some other business.”.